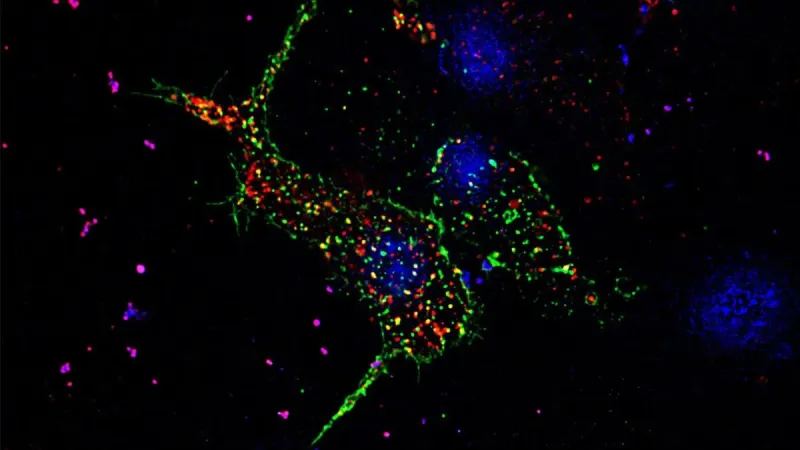
A pancreatic beta cell that shows puncta in the form of GLUT transporters in green and insulin granules in red, the nucleus of the cell in blue, taken in super-resolution under Zeiss-Elyra (Image Credit: Anuma Pallavi).
After we eat a meal, the food is converted into essential nutrients, and a rush of glucose enters our bloodstream. To keep things running smoothly, special traffic controllers in your pancreas, called beta cells, need to quickly take in this glucose and release insulin, which helps your body use that sugar for energy. But what if these traffic controllers aren't doing their job right?
A recent study from the Indian Institute of Science (IISc) has shed light on how this glucose traffic management goes wrong in Type 2 Diabetes (T2D), offering exciting new possibilities for treatment.
The Body's Glucose Gatekeepers
At the heart of this system are tiny protein gatekeepers called Glucose Transporters (GLUTs). When your blood sugar levels rise after a meal, GLUTs move to the surface of your beta cells, open their gates, and let glucose rush in. This entry of glucose is the signal for the beta cells to release insulin.
The IISc team, led by Assistant Professor Nikhil Gandasi, used imaging techniques to watch these GLUTs in action. In healthy cells, they found that when glucose levels go up, the GLUTs rapidly move to the cell surface. But this was not a one-way street. These transporters are constantly recycled, moving in and out of the cell surface through a process that involves a protein called clathrin.
However, the picture changes dramatically in beta cells from people with Type 2 Diabetes. The study found that in these cells, fewer GLUTs make it to the cell surface, and their recycling process is altered. This leads to a jam in the GLUTs movement, which further slows down glucose entry into the beta cells, resulting in less insulin being released. This is especially true for the primed insulin packages, the ones that are supposed to be released quickly right after you eat.
“Most studies have looked at what happens after glucose enters the β-cell”, explains Anuma Pallavi, a PhD student and the first author of the study. “We focused on the step before that, the actual entry of glucose, and how this is disrupted in diabetes. By understanding the dynamics of these transporters, we can identify new points to intervene and improve β-cell function.” she adds.
A New Path for Treatment?
Current treatments for diabetes often focus on how insulin works in other parts of the body, like muscles and fat. But this new research points to the beta cells themselves – specifically, how they take in glucose – as a promising new target. If we can fix the traffic management of GLUTs, we might be able to help beta cells work better and slow down the progression of diabetes.
Dr Nikhil R. Gandasi’s lab has even explored this idea before, identifying a plant-derived molecule called Pheophorbide A that can boost insulin release by interacting with glucose transporters.
“If we can restore proper GLUT trafficking, we may be able to slow down disease progression and personalise therapies based on a patient’s metabolic state. This groundbreaking work suggests that by untangling these glucose traffic jams, we might be able to pave the way for more effective and personalized treatments for Type 2 Diabetes.” concludes Dr Nikhil R Gandasi, Assistant Professor at IISc and an author of the study.
Note: Based on a press release from IISc.